Kaist
Korean

News
College of Engineering News
-
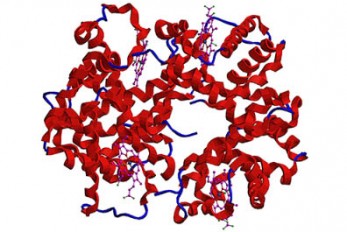
Microbial Production of a Natural Red Colorant Car..
Metabolic engineering and computer-simulated enzyme engineering led to the production of carminic acid, a natural red colorant, from bacteria for the first time < Figure: A schematic biosynthetic pathway for the production of carminic acid from glucose. Biochemical reaction analysis and computer simulation-assisted enzyme engineering was employed to identify and improve the enzymes (DnrFP217K and GtCGTV93Q/Y193F) responsible for the latter two reactions. > A research group at KAIST has engineered a bacterium capable of producing a natural red colorant, carminic acid, which is widely used for food and cosmetics. The research team reported the complete biosynthesis of carminic acid from glucose in engineered Escherichia coli. The strategies will be useful for the design and construction of biosynthetic pathways involving unknown enzymes and consequently the production of diverse industrially important natural products for the food, pharmaceutical, and cosmetic industries. Carminic acid is a natural red colorant widely being used for products such as strawberry milk and lipstick. However, carminic acid has been produced by farming cochineals, a scale insect which only grows in the region around Peru and Canary Islands, followed by complicated multi-step purification processes. Moreover, carminic acid often contains protein contaminants that cause allergies so many people are unwilling to consume products made of insect-driven colorants. On that account, manufacturers around the world are using alternative red colorants despite the fact that carminic acid is one of the most stable natural red colorants. These challenges inspired the metabolic engineering research group at KAIST to address this issue. Its members include postdoctoral researchers Dongsoo Yang and Woo Dae Jang, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering. This study entitled “Production of carminic acid by metabolically engineered Escherichia coli” was published online in the Journal of the American Chemical Society (JACS) on April 2. This research reports for the first time the development of a bacterial strain capable of producing carminic acid from glucose via metabolic engineering and computer simulation-assisted enzyme engineering. The research group optimized the type II polyketide synthase machinery to efficiently produce the precursor of carminic acid, flavokermesic acid. Since the enzymes responsible for the remaining two reactions were neither discovered nor functional, biochemical reaction analysis was performed to identify enzymes that can convert flavokermesic acid into carminic acid. Then, homology modeling and docking simulations were performed to enhance the activities of the two identified enzymes. The team could confirm that the final engineered strain could produce carminic acid directly from glucose. The C-glucosyltransferase developed in this study was found to be generally applicable for other natural products as showcased by the successful production of an additional product, aloesin, which is found in aloe leaves. “The most important part of this research is that unknown enzymes for the production of target natural products were identified and improved by biochemical reaction analyses and computer simulation-assisted enzyme engineering,” says Dr. Dongsoo Yang. He explained the development of a generally applicable C-glucosyltransferase is also useful since C-glucosylation is a relatively unexplored reaction in bacteria including Escherichia coli. Using the C-glucosyltransferase developed in this study, both carminic acid and aloesin were successfully produced from glucose. “A sustainable and insect-free method of producing carminic acid was achieved for the first time in this study. Unknown or inefficient enzymes have always been a major problem in natural product biosynthesis, and here we suggest one effective solution for solving this problem. As maintaining good health in the aging society is becoming increasingly important, we expect that the technology and strategies developed here will play pivotal roles in producing other valuable natural products of medical or nutritional importance,” said Distinguished Professor Sang Yup Lee. This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries of the Ministry of Science and ICT (MSIT) through the National Research Foundation (NRF) of Korea and the KAIST Cross-Generation Collaborative Lab project; Sang Yup Lee and Dongsoo Yang were also supported by Novo Nordisk Foundation in Denmark. Publication: Dongsoo Yang, Woo Dae Jang, and Sang Yup Lee. Production of carminic acid by metabolically engineered Escherichia coli. at the Journal of the American Chemical Society. https://doi.org.10.1021/jacs.0c12406 Profile: Sang Yup Lee, PhD Distinguished Professor leesy@kaist.ac.kr http://mbel.kaist.ac.kr Metabolic &Biomolecular Engineering National Research Laboratory Department of Chemical and Biomolecular Engineering KAIST
-
Identification of How Chemotherapy Drug Works Coul..
< Professor Yoosik Kim and PhD candidate Yongsuk Ku > The chemotherapy drug decitabine is commonly used to treat patients with blood cancers, but its response rate is somewhat low. Researchers have now identified why this is the case, opening the door to more personalized cancer therapies for those with these types of cancers, and perhaps further afield. Researchers have identified the genetic and molecular mechanisms within cells that make the chemotherapy drug decitabine—used to treat patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) —work for some patients but not others. The findings should assist clinicians in developing more patient-specific treatment strategies. The findings were published in the Proceedings of the National Academies of Science on March 30. The chemotherapy drug decitabine, also known by its brand name Dacogen, works by modifying our DNA that in turn switches on genes that stop the cancer cells from growing and replicating. However, decitabine’s response rate is somewhat low (showing improvement in just 30-35% of patients), which leaves something of a mystery as to why it works well for some patients but not for others. To find out why this happens, researchers from the KAIST investigated the molecular mediators that are involved with regulating the effects of the drug. Decitabine works to activate the production of endogenous retroviruses (ERVs), which in turn induces an immune response. ERVs are viruses that long ago inserted dormant copies of themselves into the human genome. Decitabine in essence, ‘reactivates’ these viral elements and produces double-stranded RNAs (dsRNAs) that the immune system views as a foreign body. “However, the mechanisms involved in this process, in particular how production and transport of these ERV dsRNAs were regulated within the cell were understudied,” said corresponding author Yoosik Kim, professor in the Department of Chemical and Biomolecular Engineering at KAIST. “So to explain why decitabine works in some patients but not others, we investigated what these molecular mechanisms were,” added Kim. To do so, the researchers used image-based RNA interference (RNAi) screening. This is a relatively new technique in which specific sequences within a genome are knocked out of action or “downregulated.” Large-scale screening, which can be performed in cultured cells or within live organisms, works to investigate the function of different genes. The KAIST researchers collaborated with the Institut Pasteur Korea to analyze the effect of downregulating genes that recognize ERV dsRNAs and could be involved in the cellular response to decitabine. < Schematic diagram of the molecular mechanism of decitabine. Differences in immune responses in the body according to the expression of Staufen1 and TINCR. > From these initial screening results, they performed an even more detailed downregulation screening analysis. Through the screening, they were able to identify two particular gene sequences involved in the production of an RNA-binding protein called Staufen1 and the production of a strand of RNA that does not in turn produce any proteins called TINCR that play a key regulatory role in response to the drug. Staufen1 binds directly to dsRNAs and stabilizes them in concert with the TINCR. If a patient is not producing sufficient Staufen1 and TINCR, then the dsRNA viral mimics quickly degrade before the immune system can spot them. And, crucially for cancer therapy, this means that patients with lower expression (activation) of these sequences will show inferior response to decitabine. Indeed, the researchers confirmed that MDS/AML patients with low Staufen1 and TINCR expression did not benefit from decitabine therapy. “We can now isolate patients who will not benefit from the therapy and direct them to a different type of therapy,” said first author Yongsuk Ku. “This serves as an important step toward developing a patient-specific treatment cancer strategy.” As the researchers used patient samples taken from bone marrow, the next step will be to try to develop a testing method that can identify the problem from just blood samples, which are much easier to acquire from patients. The team plans to investigate if the analysis can be extended to patients with solid tumors in addition to those with blood cancers. -Profile Professor Yoosik Kim https://qcbio.kaist.ac.kr/ Department of Chemical and Biomolecular Engineering KAIST -Publication Noncanonical immune response to the inhibition of DNA methylation by Staufen1 via stabilization of endogenous retrovirus RNAs, PNAS
-

Plasma Jets Stabilize Water to Splash Less
< High-speed shadowgraph movie of water surface deformations induced by plasma impingement. > A study by KAIST researchers revealed that an ionized gas jet blowing onto water, also known as a ‘plasma jet’, produces a more stable interaction with the water’s surface compared to a neutral gas jet. This finding reported in the April 1 issue of Nature will help improve the scientific understanding of plasma-liquid interactions and their practical applications in a wide range of industrial fields in which fluid control technology is used, including biomedical engineering, chemical production, and agriculture and food engineering. Gas jets can create dimple-like depressions in liquid surfaces, and this phenomenon is familiar to anyone who has seen the cavity produced by blowing air through a straw directly above a cup of juice. As the speed of the gas jet increases, the cavity becomes unstable and starts bubbling and splashing. “Understanding the physical properties of interactions between gases and liquids is crucial for many natural and industrial processes, such as the wind blowing over the surface of the ocean, or steelmaking methods that involve blowing oxygen over the top of molten iron,” explained Professor Wonho Choe, a physicist from KAIST and the corresponding author of the study. However, despite its scientific and practical importance, little is known about how gas-blown liquid cavities become deformed and destabilized. In this study, a group of KAIST physicists led by Professor Choe and the team’s collaborators from Chonbuk National University in Korea and the Jožef Stefan Institute in Slovenia investigated what happens when an ionized gas jet, also known as a ‘plasma jet’, is blown over water. A plasma jet is created by applying high voltage to a nozzle as gas flows through it, which causes the gas to be weakly ionized and acquire freely-moving charged particles. The research team used an optical technique combined with high-speed imaging to observe the profiles of the water surface cavities created by both neutral helium gas jets and weakly ionized helium gas jets. They also developed a computational model to mathematically explain the mechanisms behind their experimental discovery. The researchers demonstrated for the first time that an ionized gas jet has a stabilizing effect on the water’s surface. They found that certain forces exerted by the plasma jet make the water surface cavity more stable, meaning there is less bubbling and splashing compared to the cavity created by a neutral gas jet. Specifically, the study showed that the plasma jet consists of pulsed waves of gas ionization propagating along the water’s surface so-called ‘plasma bullets’ that exert more force than a neutral gas jet, making the cavity deeper without becoming destabilized. “This is the first time that this phenomenon has been reported, and our group considers this as a critical step forward in our understanding of how plasma jets interact with liquid surfaces. We next plan to expand this finding through more case studies that involve diverse plasma and liquid characteristics,” said Professor Choe. This work was supported by KAIST as part of the High-Risk and High-Return Project, the National Research Foundation of Korea (NRF), and the Slovenian Research Agency (ARRS). < Cavity formation at the water’s surface subjected to a neutral helium gas jet (left) and a weakly ionized helium gas jet (right). > Image Credit: Professor Wonho Choe, KAIST Usage Restrictions: News organizations may use or redistribute these materials, with proper attribution, as part of news coverage of this paper only. Publication: Park, S., et al. (2021) Stabilization of liquid instabilities with ionized gas jets. Nature, Vol. No. 592, Issue No. 7852, pp. 49-53. Available online at https://doi.org/10.1038/s41586-021-03359-9 Profile: Wonho Choe, Ph.D. Professor wchoe@kaist.ac.kr https://gdpl.kaist.ac.kr/ Gas Discharge Physics Laboratory (GDPL) Department of Nuclear and Quantum Engineering Department of Physics Impurity and Edge Plasma Research Center (IERC) http://kaist.ac.kr/en/ Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea (END)
-

Streamlining the Process of Materials Discovery
The materials platform M3I3 reduces the time for materials discovery by reverse engineering future materials using multiscale/multimodal imaging and machine learning of the processing-structure-properties relationship < Researchers of M3I3 Initiative at the Department of Materials Science and Enginering: Professor EunAe Cho, Professor Hye Ryung Byon, Professor Seungbum Hong, and Professor Jong Min Yuk. > Developing new materials and novel processes has continued to change the world. The M3I3 Initiative at KAIST has led to new insights into advancing materials development by implementing breakthroughs in materials imaging that have created a paradigm shift in the discovery of materials. The Initiative features the multiscale modeling and imaging of structure and property relationships and materials hierarchies combined with the latest material-processing data. The research team led by Professor Seungbum Hong analyzed the materials research projects reported by leading global institutes and research groups, and derived a quantitative model using machine learning with a scientific interpretation. This process embodies the research goal of the M3I3: Materials and Molecular Modeling, Imaging, Informatics and Integration. The researchers discussed the role of multiscale materials and molecular imaging combined with machine learning and also presented a future outlook for developments and the major challenges of M3I3. By building this model, the research team envisions creating desired sets of properties for materials and obtaining the optimum processing recipes to synthesize them. “The development of various microscopy and diffraction tools with the ability to map the structure, property, and performance of materials at multiscale levels and in real time enabled us to think that materials imaging could radically accelerate materials discovery and development,” says Professor Hong. “We plan to build an M3I3 repository of searchable structural and property maps using FAIR (Findable, Accessible, Interoperable, and Reusable) principles to standardize best practices as well as streamline the training of early career researchers.” < Figure 1. Schematic diagram of the M3I3 Flagship Project. This project aims to achieve the seamless integration of the multiscale “structure–property” and “processing–property” relationships via materials modeling, imaging, and machine learning. With the capability of artificial intelligence (AI)-guided automatic synthesis, M3I3 will provide expedited development of new materials in the near future. > One of the examples that shows the power of structure-property imaging at the nanoscale is the development of future materials for emerging nonvolatile memory devices. Specifically, the research team focused on microscopy using photons, electrons, and physical probes on the multiscale structural hierarchy, as well as structure-property relationships to enhance the performance of memory devices. “M3I3 is an algorithm for performing the reverse engineering of future materials. Reverse engineering starts by analyzing the structure and composition of cutting-edge materials or products. Once the research team determines the performance of our targeted future materials, we need to know the candidate structures and compositions for producing the future materials.” The research team has built a data-driven experimental design based on traditional NCM (nickel, cobalt, and manganese) cathode materials. With this, the research team expanded their future direction for achieving even higher discharge capacity, which can be realized via Li-rich cathodes. However, one of the major challenges was the limitation of available data that describes the Li-rich cathode properties. To mitigate this problem, the researchers proposed two solutions: First, they should build a machine-learning-guided data generator for data augmentation. Second, they would use a machine-learning method based on ‘transfer learning.’ Since the NCM cathode database shares a common feature with a Li-rich cathode, one could consider repurposing the NCM trained model for assisting the Li-rich prediction. With the pretrained model and transfer learning, the team expects to achieve outstanding predictions for Li-rich cathodes even with the small data set. With advances in experimental imaging and the availability of well-resolved information and big data, along with significant advances in high-performance computing and a worldwide thrust toward a general, collaborative, integrative, and on-demand research platform, there is a clear confluence in the required capabilities of advancing the M3I3 Initiative. Professor Hong said, “Once we succeed in using the inverse “property−structure−processing” solver to develop cathode, anode, electrolyte, and membrane materials for high energy density Li-ion batteries, we will expand our scope of materials to battery/fuel cells, aerospace, automobiles, food, medicine, and cosmetic materials.” The review was published in ACS Nano in March. This study was conducted through collaborations with Dr. Chi Hao Liow, Professor Jong Min Yuk, Professor Hye Ryung Byon, Professor Yongsoo Yang, Professor EunAe Cho, Professor Pyuck-Pa Choi, and Professor Hyuck Mo Lee at KAIST, Professor Joshua C. Agar at Lehigh University, Dr. Sergei V. Kalinin at Oak Ridge National Laboratory, Professor Peter W. Voorhees at Northwestern University, and Professor Peter Littlewood at the University of Chicago (Article title: Reducing Time to Discovery: Materials and Molecular Modeling, Imaging, Informatics, and Integration).This work was supported by the KAIST Global Singularity Research Program for 2019 and 2020. Publication: “Reducing Time to Discovery: Materials and Molecular Modeling, Imaging, Informatics and Integration,” S. Hong, C. H. Liow, J. M. Yuk, H. R. Byon, Y. Yang, E. Cho, J. Yeom, G. Park, H. Kang, S. Kim, Y. Shim, M. Na, C. Jeong, G. Hwang, H. Kim, H. Kim, S. Eom, S. Cho, H. Jun, Y. Lee, A. Baucour, K. Bang, M. Kim, S. Yun, J. Ryu, Y. Han, A. Jetybayeva, P.-P. Choi, J. C. Agar, S. V. Kalinin, P. W. Voorhees, P. Littlewood, and H. M. Lee, ACS Nano 15, 3, 3971–3995 (2021) https://doi.org/10.1021/acsnano.1c00211 Profile: Seungbum Hong, PhD Associate Professor seungbum@kaist.ac.kr http://mii.kaist.ac.kr Department of Materials Science and Engineering KAIST (END)
-

Centrifugal Multispun Nanofibers Put a New Spin on..
KAIST researchers have developed a novel nanofiber production technique called ‘centrifugal multispinning’ that will open the door for the safe and cost-effective mass production of high-performance polymer nanofibers. This new technique, which has shown up to a 300 times higher nanofiber production rate per hour than that of the conventional electrospinning method, has many potential applications including the development of face mask filters for coronavirus protection. Nanofibers make good face mask filters because their mechanical interactions with aerosol particles give them a greater ability to capture more than 90% of harmful particles such as fine dust and virus-containing droplets. The impact of the COVID-19 pandemic has further accelerated the growing demand in recent years for a better kind of face mask. A polymer nanofiber-based mask filter that can more effectively block harmful particles has also been in higher demand as the pandemic continues. ‘Electrospinning’ has been a common process used to prepare fine and uniform polymer nanofibers, but in terms of safety, cost-effectiveness, and mass production, it has several drawbacks. The electrospinning method requires a high-voltage electric field and electrically conductive target, and this hinders the safe and cost-effective mass production of polymer nanofibers. In response to this shortcoming, ‘centrifugal spinning’ that utilizes centrifugal force instead of high voltage to produce polymer nanofibers has been suggested as a safer and more cost-effective alternative to the electrospinning. Easy scalability is another advantage, as this technology only requires a rotating spinneret and a collector. However, since the existing centrifugal force-based spinning technology employs only a single rotating spinneret, productivity is limited and not much higher than that of some advanced electrospinning technologies such as ‘multi-nozzle electrospinning’ and ‘nozzleless electrospinning.’ This problem persists even when the size of the spinneret is increased. Inspired by these limitations, a research team led by Professor Do Hyun Kim from the Department of Chemical and Biomolecular Engineering at KAIST developed a centrifugal multispinning spinneret with mass-producibility, by sectioning a rotating spinneret into three sub-disks. This study was published as a front cover article of ACS Macro Letters, Volume 10, Issue 3 in March 2021. Using this new centrifugal multispinning spinneret with three sub-disks, the lead author of the paper PhD candidate Byeong Eun Kwak and his fellow researchers Hyo Jeong Yoo and Eungjun Lee demonstrated the gram-scale production of various polymer nanofibers with a maximum production rate of up to 25 grams per hour, which is approximately 300 times higher than that of the conventional electrospinning system. The production rate of up to 25 grams of polymer nanofibers per hour corresponds to the production rate of about 30 face mask filters per day in a lab-scale manufacturing system. By integrating the mass-produced polymer nanofibers into the form of a mask filter, the researchers were able to fabricate face masks that have comparable filtration performance with the KF80 and KF94 face masks that are currently available in the Korean market. The KF80 and KF94 masks have been approved by the Ministry of Food and Drug Safety of Korea to filter out at least 80% and 94% of harmful particles respectively. “When our system is scaled up from the lab scale to an industrial scale, the large-scale production of centrifugal multispun polymer nanofibers will be made possible, and the cost of polymer nanofiber-based face mask filters will also be lowered dramatically,” Kwak explained. This work was supported by the KAIST-funded Global Singularity Research Program for 2020. < Figure. (A) Schematic illustration of the centrifugal multispinning polymer nanofiber production process. (B) The polymer nanofibers spun by the system. The increase of the number of sub-disk shows the proportional enhancement of the productivity. (C) Face masks and mask filters fabricated using mass-produced nanofibers (inset). > < Image. Journal Cover > Publication: Byeong Eun Kwak, Hyo Jeong Yoo, Eungjun Lee, and Do Hyun Kim. (2021) Large-Scale Centrifugal Multispinning Production of Polymer Micro- and Nanofibers for Mask Filter Application with a Potential of Cospinning Mixed Multicomponent Fibers. ACS Macro Letters, Volume No. 10, Issue No. 3, pp. 382-388. Available online at https://doi.org/10.1021/acsmacrolett.0c00829 Profile: Do Hyun Kim, Sc.D. Professor dohyun.kim@kaist.edu http://procal.kaist.ac.kr/ Process Analysis Laboratory Department of Chemical and Biomolecular Engineering https:/kaist.ac.kr/en/ Korea Advanced Institute of Science and Technology (KAIST) Daejeon 34141, Korea (END)
-
Acoustic Graphene Plasmons Study Paves Way for Opt..
- The first images of mid-infrared optical waves compressed 1,000 times captured using a highly sensitive scattering-type scanning near-field optical microscope. - < Post-doc Researcher Sergey G. Menabde (Left) and Professor Min Seok Jang (Right) > KAIST researchers and their collaborators at home and abroad have successfully demonstrated a new methodology for direct near-field optical imaging of acoustic graphene plasmon fields. This strategy will provide a breakthrough for the practical applications of acoustic graphene plasmon platforms in next-generation, high-performance, graphene-based optoelectronic devices with enhanced light-matter interactions and lower propagation loss. It was recently demonstrated that ‘graphene plasmons’ – collective oscillations of free electrons in graphene coupled to electromagnetic waves of light – can be used to trap and compress optical waves inside a very thin dielectric layer separating graphene from a metallic sheet. In such a configuration, graphene’s conduction electrons are “reflected” in the metal, so when the light waves “push” the electrons in graphene, their image charges in metal also start to oscillate. This new type of collective electronic oscillation mode is called ‘acoustic graphene plasmon (AGP)’. The existence of AGP could previously be observed only via indirect methods such as far-field infrared spectroscopy and photocurrent mapping. This indirect observation was the price that researchers had to pay for the strong compression of optical waves inside nanometer-thin structures. It was believed that the intensity of electromagnetic fields outside the device was insufficient for direct near-field optical imaging of AGP. Challenged by these limitations, three research groups combined their efforts to bring together a unique experimental technique using advanced nanofabrication methods. Their findings were published in Nature Communications on February 19. A KAIST research team led by Professor Min Seok Jang from the School of Electrical Engineering used a highly sensitive scattering-type scanning near-field optical microscope (s-SNOM) to directly measure the optical fields of the AGP waves propagating in a nanometer-thin waveguide, visualizing thousand-fold compression of mid-infrared light for the first time. Professor Jang and a post-doc researcher in his group, Sergey G. Menabde, successfully obtained direct images of AGP waves by taking advantage of their rapidly decaying yet always present electric field above graphene. They showed that AGPs are detectable even when most of their energy is flowing inside the dielectric below the graphene. This became possible due to the ultra-smooth surfaces inside the nano-waveguides where plasmonic waves can propagate at longer distances. The AGP mode probed by the researchers was up to 2.3 times more confined and exhibited a 1.4 times higher figure of merit in terms of the normalized propagation length compared to the graphene surface plasmon under similar conditions. These ultra-smooth nanostructures of the waveguides used in the experiment were created using a template-stripping method by Professor Sang-Hyun Oh and a post-doc researcher, In-Ho Lee, from the Department of Electrical and Computer Engineering at the University of Minnesota. Professor Young Hee Lee and his researchers at the Center for Integrated Nanostructure Physics (CINAP) of the Institute of Basic Science (IBS) at Sungkyunkwan University synthesized the graphene with a monocrystalline structure, and this high-quality, large-area graphene enabled low-loss plasmonic propagation. The chemical and physical properties of many important organic molecules can be detected and evaluated by their absorption signatures in the mid-infrared spectrum. However, conventional detection methods require a large number of molecules for successful detection, whereas the ultra-compressed AGP fields can provide strong light-matter interactions at the microscopic level, thus significantly improving the detection sensitivity down to a single molecule. Furthermore, the study conducted by Professor Jang and the team demonstrated that the mid-infrared AGPs are inherently less sensitive to losses in graphene due to their fields being mostly confined within the dielectric. The research team’s reported results suggest that AGPs could become a promising platform for electrically tunable graphene-based optoelectronic devices that typically suffer from higher absorption rates in graphene such as metasurfaces, optical switches, photovoltaics, and other optoelectronic applications operating at infrared frequencies. Professor Jang said, “Our research revealed that the ultra-compressed electromagnetic fields of acoustic graphene plasmons can be directly accessed through near-field optical microscopy methods. I hope this realization will motivate other researchers to apply AGPs to various problems where strong light-matter interactions and lower propagation loss are needed.” This research was primarily funded by the Samsung Research Funding & Incubation Center of Samsung Electronics. The National Research Foundation of Korea (NRF), the U.S. National Science Foundation (NSF), Samsung Global Research Outreach (GRO) Program, and Institute for Basic Science of Korea (IBS) also supported the work. < Figure. Laser-illuminated nano-tip excites the acoustic graphene plasmon in the layer between the graphene and the gold/alumina. > Publication: Menabde, S. G., et al. (2021) Real-space imaging of acoustic plasmons in large-area graphene grown by chemical vapor deposition. Nature Communications 12, Article No. 938. Available online at https://doi.org/10.1038/s41467-021-21193-5 Profile: Min Seok Jang, MS, PhD Associate Professor jang.minseok@kaist.ac.kr http://jlab.kaist.ac.kr/ Min Seok Jang Research Group School of Electrical Engineering http://kaist.ac.kr/en/ Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea (END)
-

Distinguished Alumni Awardees 2020
The KAIST Alumni Association (KAA) announced the four recipients of the Distinguished Alumni Awards for the year 2020. The Distinguished Alumni Awards recognize graduates who have achieved outstanding accomplishments in their professional and personal lives, and who have been an inspiration to fellow alumni and students in Korea and around the globe. The four distinguished alumni of the year 2020 are listed below. President Dong-Won Kim (Department of Industrial and Systems Engineering, M.S., Class of ’82) of Jeonbuk National University is making significant contributions to the advancement of local industrial technology and the cultivation of professional personnel through outstanding research outcomes. As an educational administrator, his leadership is helping to realize long-desired projects at the university, through which he is strengthening the competitiveness of the university and the local community. Tae-Kyung Yoo (School of Electrical Engineering, M.S. and Ph.D., Class of ’83 and ’85 respectively), CEO and Chairman of Lumens, is a first-generation entrepreneur in the light emitting diode (LED) industry in Korea. He runs Lumens, a globally renowned company specializing in and leading the technological innovation of LEDs. He thereby contributes to strengthening national competitiveness and the advancement of science and technology. President Nak Kyu Lee (Department of Mechanical Engineering, M.S. and Ph.D., Class of ’85 and ’87 respectively) of the Korea Institute of Industrial Technology (KITECH) has shown excellent results in his research in which he developed core production technologies to lead the nation’s industries. He also focused on supporting on-site technologies involved in field work to apply what he developed into real production processes, and contributed greatly to improving the competitiveness of nationwide manufacturing. Hyeon-Mo Ku (School of Business and Technology Management, M.S. and Ph.D., Class of ’85 and ’93 respectively), CEO of KT Corporation, helped the nation’s leading communications company roll out the first 5G network in the world. He also strengthened national competitiveness in AI technology through ‘AI One Team,’ an industry-academic corporation project, and took the lead in developing the home-grown cloud industry. His involvement in the innovation of Korea’s ICT technology was highly recognized. Since the establishment of the award in 1992, a total of 107 alumni at home and abroad have brought distinction to the university and been honored as recipients. These recipients are playing major roles in society, and some of the notable former awardees include: KAIST President Sung-Chul Shin (2010), Samsung Electronics Vice Chairman Ki-Nam Kim (2012), Nexon Chairman Jung-Ju Kim (2007), and Krafton Chairman Byeong-Gyu Chang (2006). The President of the KAA and Advisor of Samsung Electronics, Chilhee Chung, said, “The Distinguished Alumni Awards are an honor given to alumni who have contributed to the development of the nation and society, and raised the name of their alma mater.” He added, “We can see the proud position of KAIST in the global arena just by looking at the accomplishments of our awardees.” (END)
-

Attachable Skin Monitors that Wick the Sweat Away
- A silicone membrane for wearable devices is more comfortable and breathable thanks to better-sized pores made with the help of citric acid crystals. - A new preparation technique fabricates thin, silicone-based patches that rapidly wick water away from the skin. The technique could reduce the redness and itching caused by wearable biosensors that trap sweat beneath them. The technique was developed by bioengineer and professor Young-Ho Cho and his colleagues at KAIST and reported in the journal Scientific Reports last month. “Wearable bioelectronics are becoming more attractive for the day-to-day monitoring of biological compounds found in sweat, like hormones or glucose, as well as body temperature, heart rate, and energy expenditure,” Professor Cho explained. “But currently available materials can cause skin irritation, so scientists are looking for ways to improve them,” he added. Attachable biosensors often use a silicone-based compound called polydimethylsiloxane (PDMS), as it has a relatively high water vapour transmission rate compared to other materials. Still, this rate is only two-thirds that of skin’s water evaporation rate, meaning sweat still gets trapped underneath it. Current fabrication approaches mix PDMS with beads or solutes, such as sugars or salts, and then remove them to leave pores in their place. Another technique uses gas to form pores in the material. Each technique has its disadvantages, from being expensive and complex to leaving pores of different sizes. A team of researchers led by Professor Cho from the KAIST Department of Bio and Brain Engineering was able to form small, uniform pores by crystallizing citric acid in PDMS and then removing the crystals using ethanol. The approach is significantly cheaper than using beads, and leads to 93.2% smaller and 425% more uniformly-sized pores compared to using sugar. Importantly, the membrane transmits water vapour 2.2 times faster than human skin. The team tested their membrane on human skin for seven days and found that it caused only minor redness and no itching, whereas a non-porous PDMS membrane did. Professor Cho said, “Our method could be used to fabricate porous PDMS membranes for skin-attachable devices used for daily monitoring of physiological signals.” “We next plan to modify our membrane so it can be more readily attached to and removed from skin,” he added. This work was supported by the Ministry of Trade, Industry and Energy (MOTIE) of Korea under the Alchemist Project. < Image 1. > < Image 2. > Image description: Smaller, more uniformly-sized pores are made in the PDMS membrane by mixing PDMS, toluene, citric acid, and ethanol. Toluene dilutes PDMS so it can easily mix with the other two constituents. Toluene and ethanol are then evaporated, which causes the citric acid to crystallize within the PDMS material. The mixture is placed in a mould where it solidifies into a thin film. The crystals are then removed using ethanol, leaving pores in their place. Image credit: Professor Young-Ho Cho, KAIST Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only. Publication: Yoon, S, et al. (2021) Wearable porous PDMS layer of high moisture permeability for skin trouble reduction. Scientific Reports 11, Article No. 938. Available online at https://doi.org/10.1038/s41598-020-78580-z Profile: Young-Ho Cho, Ph.D Professor mems@kaist.ac.kr https://mems.kaist.ac.kr NanoSentuating Systems Laboratory Department of Bio and Brain Engineering https://kaist.ac.kr Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea (END)
-

Wirelessly Rechargeable Soft Brain Implant Control..
Researchers have invented a smartphone-controlled soft brain implant that can be recharged wirelessly from outside the body. It enables long-term neural circuit manipulation without the need for periodic disruptive surgeries to replace the battery of the implant. Scientists believe this technology can help uncover and treat psychiatric disorders and neurodegenerative diseases such as addiction, depression, and Parkinson’s. < Optical image of a wirelessly rechargeable, soft optoelectronic system held with fingers. The device is emitting blue light from its bilateral probes. > A group of KAIST researchers and collaborators have engineered a tiny brain implant that can be wirelessly recharged from outside the body to control brain circuits for long periods of time without battery replacement. The device is constructed of ultra-soft and bio-compliant polymers to help provide long-term compatibility with tissue. Geared with micrometer-sized LEDs (equivalent to the size of a grain of salt) mounted on ultrathin probes (the thickness of a human hair), it can wirelessly manipulate target neurons in the deep brain using light. This study, led by Professor Jae-Woong Jeong, is a step forward from the wireless head-mounted implant neural device he developed in 2019. That previous version could indefinitely deliver multiple drugs and light stimulation treatment wirelessly by using a smartphone. For more, Manipulating Brain Cells by Smartphone. For the new upgraded version, the research team came up with a fully implantable, soft optoelectronic system that can be remotely and selectively controlled by a smartphone. This research was published on January 22, 2021 in Nature Communications. The new wireless charging technology addresses the limitations of current brain implants. Wireless implantable device technologies have recently become popular as alternatives to conventional tethered implants, because they help minimize stress and inflammation in freely-moving animals during brain studies, which in turn enhance the lifetime of the devices. However, such devices require either intermittent surgeries to replace discharged batteries, or special and bulky wireless power setups, which limit experimental options as well as the scalability of animal experiments. “This powerful device eliminates the need for additional painful surgeries to replace an exhausted battery in the implant, allowing seamless chronic neuromodulation,” said Professor Jeong. “We believe that the same basic technology can be applied to various types of implants, including deep brain stimulators, and cardiac and gastric pacemakers, to reduce the burden on patients for long-term use within the body.” To enable wireless battery charging and controls, researchers developed a tiny circuit that integrates a wireless energy harvester with a coil antenna and a Bluetooth low-energy chip. An alternating magnetic field can harmlessly penetrate through tissue, and generate electricity inside the device to charge the battery. Then the battery-powered Bluetooth implant delivers programmable patterns of light to brain cells using an “easy-to-use” smartphone app for real-time brain control. “This device can be operated anywhere and anytime to manipulate neural circuits, which makes it a highly versatile tool for investigating brain functions,” said lead author Choong Yeon Kim, a researcher at KAIST. Neuroscientists successfully tested these implants in rats and demonstrated their ability to suppress cocaine-induced behaviour after the rats were injected with cocaine. This was achieved by precise light stimulation of relevant target neurons in their brains using the smartphone-controlled LEDs. Furthermore, the battery in the implants could be repeatedly recharged while the rats were behaving freely, thus minimizing any physical interruption to the experiments. “Wireless battery re-charging makes experimental procedures much less complicated,” said the co-lead author Min Jeong Ku, a researcher at Yonsei University’s College of Medicine. “The fact that we can control a specific behaviour of animals, by delivering light stimulation into the brain just with a simple manipulation of smartphone app, watching freely moving animals nearby, is very interesting and stimulates a lot of imagination,” said Jeong-Hoon Kim, a professor of physiology at Yonsei University’s College of Medicine. “This technology will facilitate various avenues of brain research.” The researchers believe this brain implant technology may lead to new opportunities for brain research and therapeutic intervention to treat diseases in the brain and other organs. This work was supported by grants from the National Research Foundation of Korea and the KAIST Global Singularity Research Program. -Profile Professor Jae-Woong Jeong https://www.jeongresearch.org/ School of Electrical Engineering KAIST
-

Expanding the Biosynthetic Pathway via Retrobiosyn..
- Researchers reports a new strategy for the microbial production of multiple short-chain primary amines via retrobiosynthesis. - KAIST metabolic engineers presented the bio-based production of multiple short-chain primary amines that have a wide range of applications in chemical industries for the first time. The research team led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering designed the novel biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step. The research team verified the newly designed pathways by confirming the in vivo production of 10 short-chain primary amines by supplying the precursors. Furthermore, the platform Escherichia coli strains were metabolically engineered to produce three proof-of-concept short-chain primary amines from glucose, demonstrating the possibility of the bio-based production of diverse short-chain primary amines from renewable resources. The research team said this study expands the strategy of systematically designing biosynthetic pathways for the production of a group of related chemicals as demonstrated by multiple short-chain primary amines as examples. Currently, most of the industrial chemicals used in our daily lives are produced with petroleum-based products. However, there are several serious issues with the petroleum industry such as the depletion of fossil fuel reserves and environmental problems including global warming. To solve these problems, the sustainable production of industrial chemicals and materials is being explored with microorganisms as cell factories and renewable non-food biomass as raw materials for alternative to petroleum-based products. The engineering of these microorganisms has increasingly become more efficient and effective with the help of systems metabolic engineering – a practice of engineering the metabolism of a living organism toward the production of a desired metabolite. In this regard, the number of chemicals produced using biomass as a raw material has substantially increased. Although the scope of chemicals that are producible using microorganisms continues to expand through advances in systems metabolic engineering, the biological production of short-chain primary amines has not yet been reported despite their industrial importance. Short-chain primary amines are the chemicals that have an alkyl or aryl group in the place of a hydrogen atom in ammonia with carbon chain lengths ranging from C1 to C7. Short-chain primary amines have a wide range of applications in chemical industries, for example, as a precursor for pharmaceuticals (e.g., antidiabetic and antihypertensive drugs), agrochemicals (e.g., herbicides, fungicides and insecticides), solvents, and vulcanization accelerators for rubber and plasticizers. The market size of short-chain primary amines was estimated to be more than 4 billion US dollars in 2014. The main reason why the bio-based production of short-chain primary amines was not yet possible was due to their unknown biosynthetic pathways. Therefore, the team designed synthetic biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step. The retrobiosynthesis allowed the systematic design of a biosynthetic pathway for short-chain primary amines by using a set of biochemical reaction rules that describe chemical transformation patterns between a substrate and product molecules at an atomic level. These multiple precursors predicted for the possible biosynthesis of each short-chain primary amine were sequentially narrowed down by using the precursor selection step for efficient metabolic engineering experiments. “Our research demonstrates the possibility of the renewable production of short-chain primary amines for the first time. We are planning to increase production efficiencies of short-chain primary amines. We believe that our study will play an important role in the development of sustainable and eco-friendly bio-based industries and the reorganization of the chemical industry, which is mandatory for solving the environmental problems threating the survival of mankind,” said Professor Lee. This paper titled “Microbial production of multiple short-chain primary amines via retrobiosynthesis” was published in Nature Communications. This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea. < Biosynthetic reactions constructed in E. coli for the in vivo production of 12 SCPAs. These 12 SCPAs were the ones shown to be produced by valine decarboxylase (VlmD) in vitro (dotted boxes). Amine and carboxylic groups shown in each dotted box are presented with yellow and green circles, respectively. Reaction center carbon atoms that are subject to chemical transformations are marked with asterisks. Glycolysis is indicated with a red background, which leads to the biosynthesis of 12 amino acid precursors. Multiple reactions are presented with two or more arrows. > -Publication Dong In Kim, Tong Un Chae, Hyun Uk Kim, Woo Dae Jang, and Sang Yup Lee. Microbial production of multiple short-chain primary amines via retrobiosynthesis. Nature Communications ( https://www.nature.com/articles/s41467-020-20423-6) -Profile Distinguished Professor Sang Yup Lee leesy@kaist.ac.kr Metabolic &Biomolecular Engineering National Research Laboratory http://mbel.kaist.ac.kr Department of Chemical and Biomolecular Engineering KAIST
-

KAIST Mobile Clinic Module to Fill Negative Pressu..
Efficient versatile ready-for-rapid building system of MCM will serve as both a triage unit and bridge center in emergency medical situations < The view of ready-for-rapid-production negative pressure room called a Mobile Clinic Module (MCM) developed by KAIST. > A team from KAIST has developed a low-cost and ready-for-rapid-production negative pressure room called a Mobile Clinic Module (MCM). The MCM is expandable, moveable, and easy to store through a combination of negative pressure frames, air tents, and multi-function panels. The MCM expects to quickly meet the high demand for negative pressure beds in the nation and eventually many other countries where the third wave of COVID-19 is raging. The module is now ready to be rolled out after a three-week test period at the Korea Cancer Center Hospital. Professor Tek-Jin Nam’s team swung into action, rapidly working together with researchers, engineers with expertise in mechanical design, and a team of clinical doctors to complete the MCM as one of KAIST’s New Deal R&D initiatives launched last July. Professor Nam cites ‘expandability’ as the key feature of the MCM. Eventually, it will serve as both a triage unit and bridge center in emergency medical situations. “The module is a very efficient and versatile unit building system. It takes approximately two hours to build the basic MCM unit, which comprises four negative pressure bed rooms, nurse’s station, locker room, and treatment room. We believe this will significantly contribute to relieving the drastic need for negative pressure beds and provide a place for monitoring patients with moderate symptoms,” said Professor Nam. “It will also be helpful for managing less-severe patients who need to be monitored daily in quarantined rooms or as bridge stations where on-site medical staff can provide treatment and daily monitoring before hospitalization. These wards can be efficiently deployed either inside or outside existing hospitals.” The research team specially designed the negative pressure frame to ensure safety level A for the negative pressure room, which is made of a multi-function panel wall and roofed with an air tent. The multi-function panels can hold medical appliances such as ventilators, oxygen and bio-signal monitors. Positive air pressure devices supply fresh air from outside the tent. An air pump and controller maintain air beam pressure, while filtering exhausted air. An internal air information monitoring system efficiently controls room air pressure and purifies the air. While a conventional negative pressure bed is reported to cost approximately 3.5 billion KRW (50 billion won for a ward), this module is estimated to cost 0.75 billion won each (10 billion won for a ward), cutting the costs by approximately 80%. The MCM is designed to be easily transported and relocated due to its volume, weight, and maintainability. This module requires only one-fourth of the volume of existing wards and takes up approximately 40% of their weight. The unit can be transported in a 40-foot container truck. “We believe this will significantly contribute to relieving the drastic need for negative pressure beds and provide a place for monitoring patients with moderate symptoms. We look forward to the MCM upgrading epidemic management resources around the world.” Professor Nam’s team is also developing antiviral solutions and devices such as protective gear, sterilizers, and test kits under the KAIST New Deal R&D Initiative that was launched to promptly and proactively respond to the epidemic. More than 45 faculty members and researchers at KAIST are collaborating with industry and clinical hospitals to develop the antiviral technology that will improve preventive measures, diagnoses, and treatment.
-
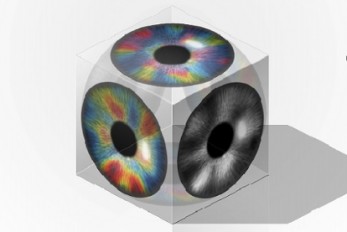
A Biological Strategy Reveals How Efficient Brain ..
- A KAIST team’s mathematical modelling shows that the topographic tiling of cortical maps originates from bottom-up projections from the periphery. - Researchers have explained how the regularly structured topographic maps in the visual cortex of the brain could arise spontaneously to efficiently process visual information. This research provides a new framework for understanding functional architectures in the visual cortex during early developmental stages. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has demonstrated that the orthogonal organization of retinal mosaics in the periphery is mirrored onto the primary visual cortex and initiates the clustered topography of higher visual areas in the brain. This new finding provides advanced insights into the mechanisms underlying a biological strategy of brain circuitry for the efficient tiling of sensory modules. The study was published in Cell Reports on January 5. In higher mammals, the primary visual cortex is organized into various functional maps for neural tuning such as ocular dominance, orientation selectivity, and spatial frequency selectivity. Correlations between the topographies of different maps have been observed, implying their systematic organizations for the efficient tiling of sensory modules across cortical areas. These observations have suggested that a common principle for developing individual functional maps may exist. However, it has remained unclear how such topographical organizations could arise spontaneously in the primary visual cortex of various species. The research team found that the orthogonal organization in the primary visual cortex of the brain originates from the spatial organization in bottom-up feedforward projections. The team showed that an orthogonal relationship among sensory modules already exists in the retinal mosaics, and that this is mirrored onto the primary visual cortex to initiate the clustered topography. By analyzing the retinal ganglion cell mosaics data in cats and monkeys, the researchers found that the structure of ON-OFF feedforward afferents is organized into a topographic tiling, analogous to the orthogonal intersection of cortical tuning maps. Furthermore, the team’s analysis of previously published data collected on cats also showed that the ocular dominance, orientation selectivity, and spatial frequency selectivity in the primary visual cortex are correlated with the spatial profiles of the retinal inputs, implying that efficient tiling of cortical domains can originate from the regularly structured retinal patterns. Professor Paik said, “Our study suggests that the structure of the periphery with simple feedforward wiring can provide the basis for a mechanism by which the early visual circuitry is assembled.” He continued, “This is the first report that spatially organized retinal inputs from the periphery provide a common blueprint for multi-modal sensory modules in the visual cortex during the early developmental stages. Our findings would make a significant impact on our understanding the developmental strategy of brain circuitry for efficient sensory information processing.” This work was supported by the National Research Foundation of Korea (NRF). < Figure 1. The image depicts the retinal origin of functional maps of neural tuning in visual cortex. > < Figure 2. The image depicts the orthogonal intersection of cortical tuning maps that are initiated by the topographic tiling of retinal ganglion cell mosaics. > < Figure 3. The regularly structured retinal circuits provide a blueprint of the clustered topography of multiple tuning maps in the primary visual cortex. > Image credit: Professor Se-Bum Paik, KAIST Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only. Publication: Song, M, et al. (2021) Projection of orthogonal tiling from the retina to the visual cortex. Cell Reports 34, 108581. Available online at https://doi.org/10.1016/j.celrep.2020.108581 Profile: Se-Bum Paik, Ph.D Assistant Professor sbpaik@kaist.ac.kr http://vs.kaist.ac.kr/ VSNN Laboratory Department of Bio and Brain Engineering Program of Brain and Cognitive Engineering http://kaist.ac.kr Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea Profile: Min Song Ph.D. Candidate night@kaist.ac.kr Program of Brain and Cognitive Engineering Profile: Jaeson Jang, Ph.D. Researcher jaesonjang@kaist.ac.kr Department of Bio and Brain Engineering, KAIST (END)

